🔒 Protect your brand with India's trusted Trademark Experts. Start your dream business with us!
GMP Certification Online in India
LegalTax offers end-to-end GMP (Good Manufacturing Practice) certification services for businesses across India. Our team ensures your manufacturing processes comply with national and international quality standards, helping you produce safe and high-quality products.
From documentation and process evaluation to inspection support and final certification, we guide you at every step to achieve GMP compliance efficiently. With GMP certification, your business gains credibility, enhances customer trust, and meets regulatory requirements, enabling growth and market expansion.
Get your GMP Certification Today
How to Start a GMP Registration

Overview
GMP stands for Good Manufacturing Practices, and it describes the manufacturing, testing, and overall quality control of pharmaceutical products. GMP certification ensures that the products comply with quality standard norms.
The Joint Commissioner is authorized by the Commissioner of Food and Drug Administration to sign and issue certificates under the WHO-GMP Certification Scheme. GMP certification covers documentation, record keeping, personnel qualification, sanitation, equipment verification, complaint handling, and process verification.
Purpose of Good Manufacturing Practices
The main objective of GMP is to reduce risks in pharmaceutical production. Risks include:
- Unforeseen impurities causing serious health effects.
- Mislabeling leading to wrong medicine intake.
- Incorrect ingredient amounts causing ineffective or harmful treatment.
GMP covers all aspects of production, from raw materials to equipment and employee hygiene, ensuring high-quality finished products.
Benefits of GMP Registration
- Empowers manufacturers to maintain high-quality production
- Identifies manufacturing and management issues timely
- Ensures compliance with laws and regulations
- Improves credibility and public image
- Reduces safety risks
- Boosts consumer confidence
- Reduces operating costs and penalties
- Promotes export opportunities
- Reduces duplicate inspections
- Saves money
Documents Required for GMP Certification
- Applicant details: name, address, contact info
- Copy of manufacturing license
- List of approved products
- Site master file (WHO TRS823)
- Data on ready formulations (Master formula, manufacturing process, product specifications)
- Process verification reports
- Technical staff details and qualifications
- Tools and equipment list
- SOPs and STPs
- Manufacturing plant layout
- Product Summary Sheet
- Proof of safety and effectiveness
GMP Certification Process
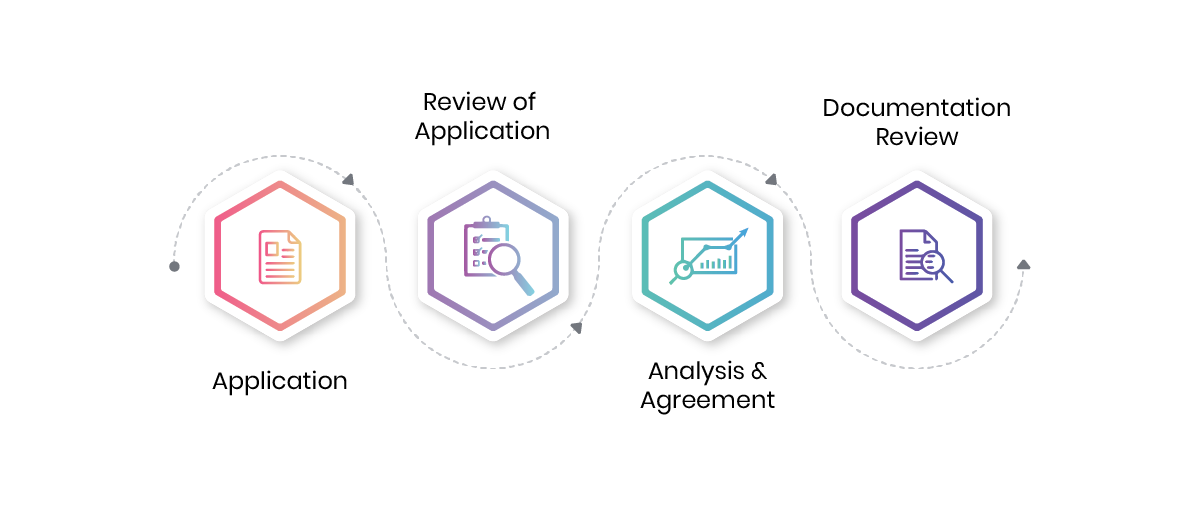
- Application: Submit required information and documents.
- Review: Ensure all continuity and compliance requirements are met.
- Gap Analysis: Identify areas for compliance improvement.
- Documentation Review: Check for proper documentation and standards.
- Stage-1 Audit: Online verification and corrective actions.
- Stage-2 Audit: Inspector checks, corrective actions, certification issuance, and surveillance audits.
Consequences of GMP Non-Compliance
- Damage to reputation and goodwill
- Loss of consumer confidence
- Legal prosecution
- License cancellations
- Allegations of fraud
Basic Principles of GMP
- Maintain a clean and hygienic manufacturing environment
- Control and define all manufacturing processes
- Proper documentation of all procedures
- Investigate deviations and non-compliance
- Ensure staff training and hygiene
Why Choose LegalTax?
LegalTax provides expert guidance and assistance for GMP certification, ensuring compliance, smooth processes, and cost-effective solutions. Clients can track progress and receive professional support throughout the certification process.